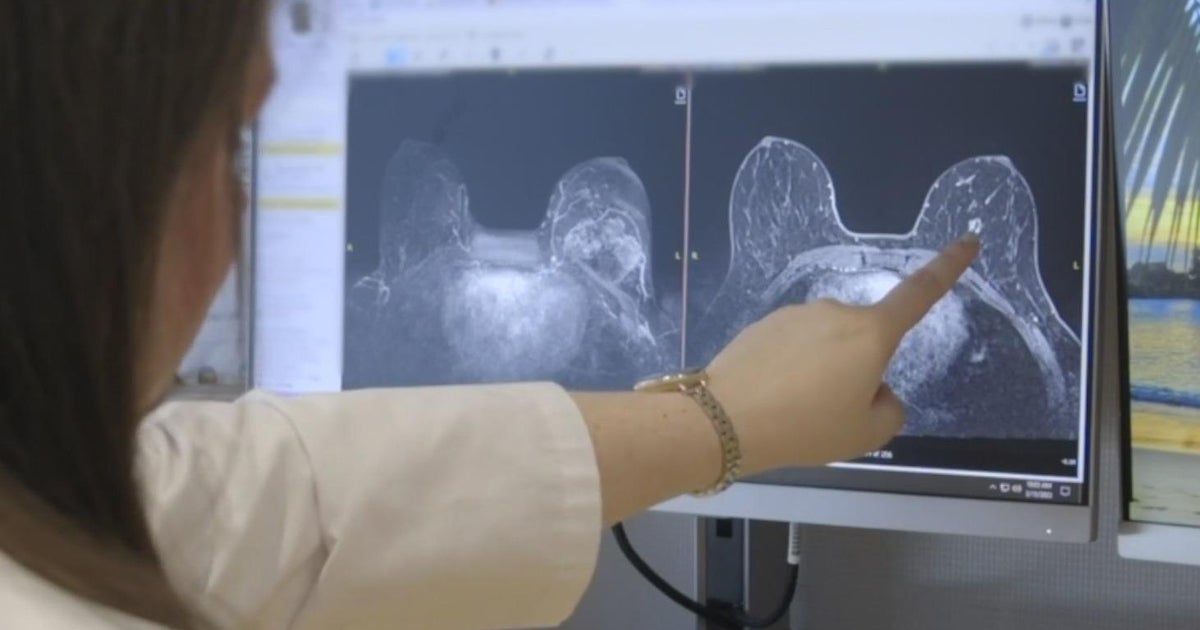
SACRAMENTO — An estimated one in eight women will develop breast cancer in their lifetime, killing on average 42,000 women a year in the United States.
What if there was a vaccine that would significantly lower each woman’s chance of ever getting it in the first place? In that question lies what could be the answer to one day eliminating the deadly disease.
A groundbreaking vaccine created through decades of research at the Cleveland Clinic and developed by Anixa Biosciences in San Jose, California is driving innovation by targeting triple-negative breast cancer, the disease’s deadliest and most aggressive form.
“This vaccine could potentially eliminate breast cancer,” said Dr. Amit Kumar, Anixa CEO.
The vaccine’s findings from its first trial with 16 women were published Wednesday, with each participant reporting no bad side effects and no resurgence of their cancer so far.
Jennifer Davis, a brave woman from small-town Ohio, was the first woman in the world to get the vaccine in October 2021.
“This is how we advance medicine. It’s important to be a part of those things,” Davis said. “I am just beyond grateful.”
When Davis heard the dreaded words “you have cancer” in September 2018, it came six months after her first alert to an abnormality on a routine mammogram and ultrasound.
At the time, her biopsy turned up negative for cancer.
“I really wanted to believe everything was OK, but I knew something wasn’t right,” Davis said.
At 41 years old, she had no history of cancer. Still, she could feel a lump growing and decided to go for a second opinion and a second biopsy.
She was diagnosed with triple-negative breast cancer. Her mind instantly went to her family and three children.
“It was very hard to tell them and try and be strong for them,” Davis said. “With triple negative, there is nothing for us to take — no pill or anything to prevent recurrence. The rate is high and outcomes are poor if it does come back.”
After chemotherapy, a double mastectomy and radiation, Davis was finally free of cancer, but she was not free of the fear that lingers.
“I was always nervous and afraid of it coming back,” she said.
So when she learned of an experimental vaccine trial while receiving her cancer care at the Cleveland Clinic, she thought, “What do I have to lose?”
“It was something that was going to give me peace of mind,” Davis said. “If this could work for me, then I wouldn’t have to worry about a recurrence.”
She became the first woman in the world to take the breast cancer vaccine.
A registered nurse herself, what eased her mind was the fact that in years of trials in animals, there had been no cancer recurrence and no anaphylactic reaction.
“That was all I needed to hear,” said Davis, who reports that in the two years since taking the vaccine, she has never felt better.
The vaccine has been studied for more than two decades at the Cleveland Clinic, pioneered by pre-clinical research led by the late Dr. Vincent Tuohy.
Inspired by this and what it could mean for the future of cancer diagnosis, Dr. Kumar approached the clinic about developing the vaccine.
“I looked at it and I saw the vision,” Kumar said.
So how does it work?
“Is it, in essence, teaching your body not to grow a tumor?” CBS13 reporter Ashley Sharp asked.
“That’s exactly right. It’s teaching your body to destroy the cells that can grow a tumor,” Kumar said.
If a virus shows up in the body, the immune system teaches itself how to destroy it, knowing, easily, which cells are bad.
In cancer, it is more difficult, Dr. Kumar explained.
“All of the cells that become cancerous in your body came from normal, healthy cells,” Kumar said. “The difference is not big, so the immune system has a harder time recognizing a cancer cell and distinguishing it from a healthy cell.”
According to the Cleveland Clinic, the vaccine works by targeting a lactation protein called α-lactalbumin, which is no longer found after lactation in normal, aging tissues. It is, however, present in most triple-negative breast cancer patients. If breast cancer develops, the vaccine is designed to instruct the immune system to attack the tumor and keep it from growing entirely.
“The results are incredibly promising,” Kumar said. “The vision is one day to be able to give this to any woman who wants to prevent cancer from ever occurring in her body. It’s a small step and we have many more steps to go, but it’s incredible if we can make this happen.”
It’s a promising find for the future of fighting cancer that started with one woman but hopefully ends with every woman.
“The bigger picture of this is overwhelming for me,” Davis said.
The second vaccine trial is set to start in 2024, this time with 600 women instead of 16. This study will be on a much larger scale, where half the women will get the vaccine and the other half will get a placebo.
The hope is that within five years, they can get FDA approval to distribute the vaccine to the public.